Introducing the fourth state of matter
We encounter the usual three states of matter: solid, liquid and gas regularly in our daily life. However, little is known about plasma, the so-called fourth state of matter, even though 99% of visible matter in our universe is made out of it. Even less known is the fact that plasma can be produced artificially and has many different uses. The potential of this all-rounder in the field of material cleaning and surface pretreatment for downstream processes, e.g. bonding, painting, printing or coating, has made it indispensable in many industries and its processes, including automobile engineering, e-mobility, transport, electronics manufacturing, packaging technology, consumer goods, life sciences, textiles and new forms of energy.
Topics on this page:
What exactly is plasma? Plasma on surfaces Characterization of plasma
Modification of surfaces Industrial plasma pretreatment Learn more
What exactly is plasma?
A state of matter is a qualitative state of substances that depends on temperature and pressure. Atomic bonds become more unstable as temperature rises. A substance can go from the first into the second and then into the third state: solids become liquids, and liquids become gases. If a gas receives more energy, it releases electrons and becomes ionized. Plasma is ionized gas that contains ions, free electrons, excited molecules, radicals, and molecular fragments. You can find plasma everywhere in the universe.
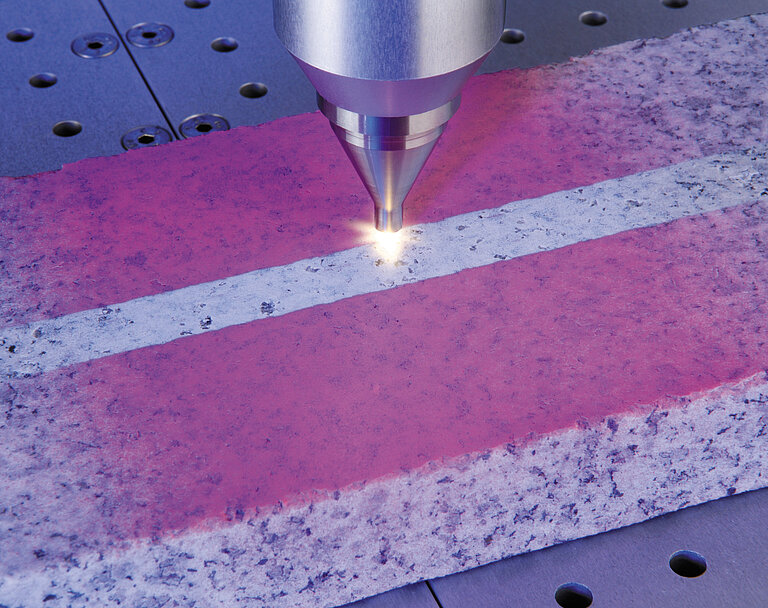
Nearly all visible matter is made of plasma: the sun, gas clouds, stars and galaxies. In nature, we can observe plasma in the form of northern lights , when the air molecules of the Earth's atmosphere, excited by the plasma of the solar winds, glows in green, blue, red, and violet. Plasma is also present in sparks, lightning and flames. It can also be produced artificially in the lab by strong gas discharges in cylindrical or tubular chambers. These discharges cause high temperatures that vaporize all substances and ionize neutral atoms and molecules which create ions and free electrons.


Plasma on surfaces: a myriad of new properties
Plasma is matter with a high, unstable energy levels which is characterized, among other things, by a high electrical conductivity. Chemically, plasma is very reactive and can interact with surfaces, liquids or microorganisms. If it comes into contact with solid materials such as plastic, glass or metal, it alters the surface energy, e.g. from hydrophobic to hydrophilic. Therefore, important surface properties are changed, e.g. the adhesiveness and wettability of surfaces. This makes it possible to use completely new (even non-polar) materials and environmentally-friendly, solvent-free (VOC-free) paints and adhesives industrially. Today, many chemical surface treatment processes can be replaced with plasma treatment: with plasma technology, surfaces can be pretreated in an environmentally friendly manner — without any solvents (VOC) or CO2 emissions. There is no need for chemicals, primers or adhesion promoters when using plasma technology.
How are plasmas characterized?
The measuring variables that particularly characterize plasma are electron temperature and emission of various species excited in the ultraviolet and visible range.
The only way to pretreat even very thermally sensitive plastics under atmospheric pressure without damage is by using high electron temperature and low ion temperature.
The luminous phenomena (optical emissions) of a relaxing* plasma can be detected with the use of optical emission spectroscopy (OES). To do this, characteristic emission bands of the species excited in the plasma in the visible, and especially in the ultraviolet, range of the spectrum are transmitted by an optical fiber to the evaluation electronics and then further processed with special software. The process monitoring components of the Plasmatreat systems operate according to this principle of optical monitoring. This allows for uniform quality over the entire plasma process.
* Relaxing: The transition of plasma into its basic state. In this process, the excitation energy that was previously supplied is released into the environment in the form of light.

When plasma meets surfaces, new properties emerge
Plasma characterises matter at a high, unstable energy level and is distinguished, among other things, by great electrical conductivity. Plasma is chemically very reactive and can interact with surfaces, liquids or microorganisms. If it comes into contact with solid materials, e.g. plastic or metal, the applied plasma energy changes important properties of these surfaces, e.g. the surface energy.
This modification of surfaces can be used in a targeted manner:
Example for microfine cleaning of metal and glass: When plasma hits metal or glass, it causes microfine cleaning. Even highly sensitive surfaces can be completely freed of undesirable substances. Fine cleaning with plasma removes even the smallest dust particles from the treated substrate. Due to the chemical-physical reaction in the nano range, high-quality, precisely defined surfaces are achieved in this way, which have the best prerequisites for further processing. The fine cleaning of surfaces before bonding, coating, varnishing or printing subsequently enables the use of modern solvent-free or water-based systems. Additional pretreatment with chemical primers or mechanical processes, e.g. brushing, can be completely dispensed with. This means that emissions of VOCs (volatile organic compounds) can be avoided during production. Cleaning with atmospheric plasma is also a dry process. In industrial processes, this offers the great advantage of being able to process all materials immediately - and thus saves an enormous amount of time.
Example for an activated surface and better wettability of plastics: Plasma pre-treatment can also change the properties of material surfaces when they come into contact with a wide variety of plastics: Oxygen- and nitrogen-containing groupings are introduced into the surfaces of the mostly non-polar plastics through contact with plasma. These significantly increase the surface energy - a so-called activation takes place. This ensures a significantly improved wettability of the substrate, which in turn leads to an increase in adhesion. Adhesives, paints or lacquers therefore adhere much better and more durably to the pre-treated surface without conventional pretreatments, e.g. flame treatment or the use of environmentally harmful chemicals.